NEWS CENTER
- 口罩等医疗物资出口白名
- 浙江一外贸企业因做跨境
- 2018元旦快乐
- 海运DDP、DDU、DAP有什么区
- 在海运专线中海运提单查
- 什么是转港贸易,第三国
- 离岸贸易转口贸易的之间
- 进出口报关,客人要求发
- 出口到伊朗需注意,你的
- 笔记本电脑,电子产品可
- 进出口报关,AEO企业编码
- 服装的出口关税税率是多
- 如何利用转口贸易应对美
- 最新取得国外认证标准或
- 进出口报关,出口美国的
- 海运篇:截港、截单、截
- 货代朋友圈被这张图刷屏
- 海关报关是流程,查验才
- 受疫情影响,DHL、UPS停运
- 出口报关错误影响退税的
- 2018最新<美国公布建议
- 最新取得国外认证标准或
- KN95能出口吗?KN95口罩重
- 中美贸易战加征收关税产
- 香港恒生12月底将关闭过
- 【重要】今天起,新版报
- 朋友们注意了,伊拉克发
- 科特迪瓦海关宣布从7月
- 中美贸易战,美国启动
- 国际物流,跨境电商eBay退
- 什么是反倾销?如何应对
- 什么是是转口贸易,第三
- 转港贸易是什么?又要如
- 国际快递,进出口报关个
- 第三国转口,转口贸易的
- 关于最近出现冒充我公司
- 国际物流运输包装要求你
- 国际海运,散货拼箱限重
- 巨东跨境电商物流亚马逊
- 亚马逊FBA头程运输费用怎
作者:巨东物流 | 发布时间:2020-06-16 12:01 | 来源:巨东资讯 关注量:
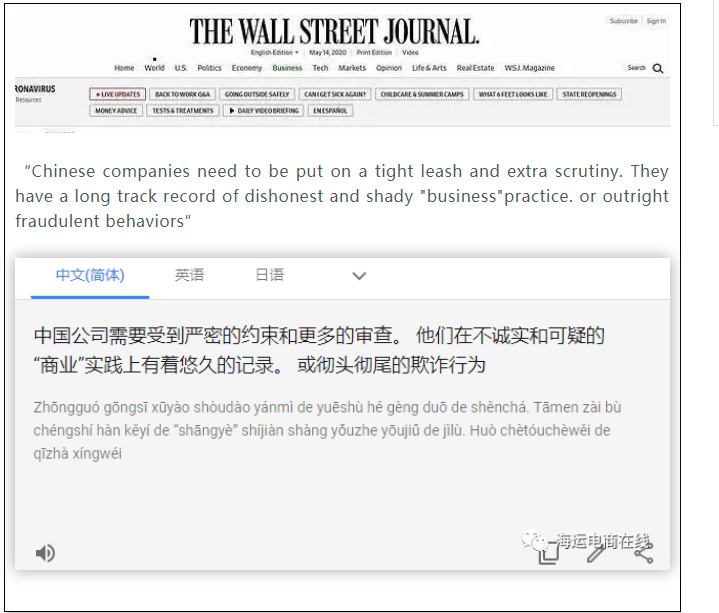
继此前对中国口罩企业的过滤效果发难后
对中国口罩企业此前FDA注册的某些不合规
成了美方新的清算方向

华尔街日报(WSJ)一份分析显示,超过1,300家中国医疗器材公司,在2019年冠状病毒疾病(COVID-19)肆虐期间,据称将德拉瓦州(Delaware)一个实体列为其在美国的代表,但该实体使用的,却是假地址和无人接听的电话号码。
华尔街日报报导,所有外国医疗器材制造商都被要求在美国必须有一个代理机构或称代表处,此代表处必须拥有真实地址,且在营业时间有人值班接听电话。
这些公司的美国代表,是食品暨药物管理局(FDA)与这些海外公司之间的联络人,以便协调检查、召回或应付其他紧急需求。
华尔街日报的最新报道披露,仅仅从其调查的一家公司名为CCTC Service Inc的情况显示:至少1,300家注册的中国公司,将CCTC Service Inc.列为他们的美国代表。但根据公司纪录资料库,这家以CCTC Service Inc为名的公司在美国并不存在。
CCTC声称所在地址,是位于德拉瓦州威明顿(Wilmington)的一栋3房砖造房屋,该地址的房客和房东说,他们对CCTC或任何中国公司一无所知。
华尔街日报指出,CCTC的详细注册讯息源自一家名为Shenzhen CCT Testing Technology Co.的检测企业。
这家总部位于深圳的公司指出,它就是将CCTC列为那么多中国公司在美国代理人的幕后推手。
来自这家检测公司的资深经理Tony Mo在提到CCTC的时候说:“我们当然不是假的公司”。
但他也承认,这家被列在FDA众多纪录中的CCTC,从未正式在美国成立。

在FDA匆忙应对疫情大流行过程中,FDA的紧急使用授权计划出现监管漏洞。华尔街日报的调查结果,凸显FDA医疗器材资料库的瑕疵,防护口罩的销售商通常会援引FDA医疗器材资料库证明自身的合法性。
FDA拒绝就CCTC置评。一名发言人表示,FDA的注册资料库对大众来说是一项有用工具,但列入该资料库不代表获得FDA批准,该机构也未认证资料库中的注册资讯。
虽然FDA没有置评,但华尔街日报发布事件报道后,一些美国网友带歧视性的留言令人愤怒:
从目前情况来看,随着疫情逐步走稳,对中国口罩企业的清算将会是接下来一段时间的主旋律,口罩过滤效果是否达标,FDA注册信息和流程是否合规,甚至售价是否合理,这些都将是很多中国口罩企业要过的关,一旦过关失败,诉讼以及后续的高额罚款或罚金将不可避免。
以下是中国第一家口罩企业收到的美国起诉书,关于起诉的原因和即将面临的追责,值得出口美国市场的口罩企业可以仔细研究。
中国口罩企业-King Year Packaging and Printing Co. Ltd.(金年包装印刷有限公司)收到的美方起诉书原文:
UNITED STATES DISTRICT COURT
EASTERN DISTRICT OF NEW YORK
---------------------------------------------------X
UNITED STATES OF AMERICA
- against -
KING YEAR PRINTING AND
PACKAGING CO., LTD.,
Defendant.
--------------------------------------------------X
COMPLAINT AND AFFIDAVIT IN
SUPPORT OF APPLICATION FOR
SUMMONS .
(T. 21 U.S.C. §§ 331(a), 333(a)(2), and 18
U.S.C. §§ 1001(a), 2)
No. 20-MJ-416
EASTERN DISTRICT OF NEW YORK, SS:
DONALD PEARLMAN, being duly sworn, deposes and says that he is a
Special Agent with the U.S. Food and Drug Administration, Office of Criminal Investigations
(“FDA-OCI”), duly appointed according to law and acting as such.
Counts One Through Three: Introduction of Misbranded Devices into Interstate Commerce
1. On or about the dates set forth below, in the Eastern District of New York
and elsewhere, the defendant KING YEAR PRINTING AND PACKAGING CO., LTD.
(hereinafter also referred to as “KING YEAR”), with intent to defraud and mislead, did introduce
and deliver for introduction, and cause to be introduced and delivered for introduction into
interstate commerce devices that were misbranded within the meaning of Title 21, United States
Code, Section 352(a)(1), specifically self-breathing filtration particle-preventive respirators
whose labeling falsely included the NIOSH logo despite the respirators not being NIOSH
approved, and “N95” markings and a test report showing compliance with the N95 standard
despite the respirators not meeting the minimum standard for N95 respirators, in the approximate
quantities set forth below.
- 2 -
Count Approximate
Date
Approximate Quantity of
Respirators
1 4/6/20 95,200
2 4/18/20 300,000
3 4/21/20 100,000
(Title 21, United States Code, Sections 331(a) and 333(a)(2), and Title 18, United States
Code, Sections 2 and 3551 et seq.)
Count Four: False Statements
2. On or about April 10, 2020, in the District of Maryland and elsewhere, the
defendant, KING YEAR PRINTING AND PACKAGING CO., LTD., by and through the acts of
its agents and employees who were acting within the scope of their agency and employment and
with intent to benefit KING YEAR, in a matter within the jurisdiction of the executive branch of
the United States, namely, the U.S. Food and Drug Administration, did knowingly and willfully
(a) falsify, conceal, and cover up by trick, scheme, and device, certain material facts; (b) make
materially false, fictitious, and fraudulent statements and representations; and (c) make and use
false writings and documents knowing them to contain materially false, fictitious, and fraudulent
statements and entries.
(Title 18, United States Code, Sections 1001(a), 2, and 3551 et seq.)
3. The source of your deponent’s information and the grounds for his
belief are as follows:
4. I am a Special Agent with FDA-OCI. I am aware of the facts contained
herein based upon my own investigation and based upon interviews and briefings with other law
enforcement officers who have participated in this investigation. I also have participated in
- 3 -
witness interviews and reviewed other evidence, including email and text message
communications, telephone toll records, and publicly-available reports. Because this affidavit is
being submitted for the limited purpose of establishing probable cause, I have not included each
and every fact known to me concerning this investigation. I have set forth only the facts which I
believe are necessary to establish probable cause. Unless specifically indicated, all conversations
and statements described in this affidavit are related in substance and in part.
Relevant Entities and Individuals
5. Defendant KING YEAR PRINTING AND PACKAGING CO., LTD. was
a company located in Guangdong province in the People’s Republic of China (“PRC”) that was
engaged in the business of manufacturing, exporting, and distributing products, including
personal protective equipment (“PPE”), to the United States and throughout the world.
6. Individual-1 was a U.S. citizen residing in the PRC who brokered deals for
pharmaceutical and medical products from companies located in the PRC. As COVID-19 spread
in the PRC and throughout the world, Individual-1 entered the business of buying, selling, and
importing PPE into the United States. Individual-1 held himself out to buyers in the United
States as having connections to Chinese pharmaceutical companies, medical equipment
manufacturers, provincial governmental leaders, and central government ministry leaders,
including the Ministry of Health.
7. Individual-2 was a relative of Individual-1. Individual-2 was a U.S. citizen
who resided in Salt Lake City, Utah. Individual-2 was a licensed acupuncturist and the owner
and operator of Company-1.
8. Company-1 was a Utah corporation located in West Valley City, Utah. Its
primary business involved the import and export of supplements and raw materials from the PRC.
- 4 -
9. Individual-3 was a U.S. citizen who resided in Lincoln University,
Pennsylvania. Individual-3 owned and operated Company-2.
10. Company-2 was a Delaware limited liability company located in Lincoln
University, Pennsylvania, with warehouse space in Newark, Delaware. Prior to the COVID-19
pandemic, Company-2 imported and distributed hazmat protective suits from a manufacturer in
Italy. As COVID-19 spread in the United States, Individual-3 used Company-2 to accumulate
large quantities of PPE, including facemasks and respirators, and resell them to private brokers
and various governmental entities, medical providers, and first responders, often at substantial
mark-ups over the prices he paid to acquire the PPE.
11. Company-3 was a Chinese medicine manufacturer, importer, and
distributor located in Guangdong province in the PRC.
12. Company-4 was a Chinese import and export broker located in Jiangsu
province in the PRC.
Overview
13. Between on or about April 6, 2020 and on or about April 21, 2020, in the
midst of the COVID-19 pandemic, defendant KING YEAR PRINTING AND PACKAGING CO.
LTD. exported approximately 495,200 defective and misbranded “N95” respirators (the “Subject
Respirators”) from the PRC to the United States. The defendant KING YEAR falsely labeled the
Subject Respirators with the intent to defraud U.S. consumers, including medical providers and
state and local governments, into believing they were buying N95 respirators approved, cleared,
or otherwise authorized by the U.S. Food and Drug Administration (“FDA”) and National
Institute for Occupational Safety and Health (“NIOSH”) of the Centers for Disease Control and
Prevention (“CDC”). In fact, the Subject Respirators were neither FDA-approved, cleared, nor
- 5 -
authorized nor NIOSH-approved. And the Subject Respirators did not perform at the promised
minimum 95% filtration efficiency level. Nevertheless, the defendant KING YEAR attempted to
cover up the poor quality of the Subject Respirators in various ways – by stamping FDA and
NIOSH logos on its packaging, by embroidering each Subject Respirator with the label, “N95,”
by procuring and disseminating false documents attesting to the authenticity of the Subject
Respirators, by filing a false registration document claiming the masks were NIOSH-approved,
and by using a fictitious corporation as its U.S. agent in registration documents filed with the
FDA.
The Global COVID-19 Pandemic
14. In December 2019, a novel coronavirus, SARS-CoV-2, was first detected
in Wuhan Province of the PRC, causing outbreaks of the coronavirus disease 2019 (“COVID19”) that have since spread globally. On January 31, 2020, the Secretary of Health and Human
Services declared a national public health emergency under 42 U.S.C. § 247d as a result of the
spread of COVID-19 to and within the United States. On March 11, 2020, the Director-General
of the World Health Organization characterized COVID-19 as a pandemic. On March 13, 2020,
the President of the United States issued Proclamation 9994 declaring a national emergency as a
result of the rapid spread of COVID-19 within the United States.
15. According to the CDC, the virus that causes COVID-19 spreads through
respiratory droplets produced when an infected person coughs or sneezes. Droplets can land in
the mouths, noses, or eyes of people who are nearby or possibly be inhaled into the lungs of those
within close proximity.
16. Accordingly, the CDC has issued guidance to health care providers
recommending that they wear PPE, such as N95 respirators, to prevent the coronavirus from
- 6 -
being transmitted by infected patients to healthcare providers. The CDC also has encouraged the
public to use face coverings to prevent the spread of the virus. To that end, on or about April 15,
2020, New York Governor Andrew M. Cuomo issued an executive order requiring all people in
New York to wear face coverings in public.
The Surge in Demand for N95 Respirators
17. According to CDC guidance, face masks are used by the general public and
by healthcare professionals as source control, which refers to the use of a facemask or cloth face
covering over the mouth and nose to contain that individual’s respiratory secretions to help
prevent transmission from infected individuals who may or may not have symptoms of COVID19. Face masks sometimes contain ear loops, which are not designed to form a seal around the
nose and mouth.
18. A surgical mask is a loose-fitting, disposable device that creates a physical
barrier between the mouth and nose of the wearer and potential contaminants in the immediate
environment. Surgical masks are not designed to form a seal around the nose and mouth. A
picture of a surgical mask is attached below:
19. An N95 respirator is a disposable half-mask filtering facepiece respirator
that covers the user’s airway (nose and mouth) and offers protection from particulate materials at
an N95 filtration efficiency level per 42 CFR 84.170. N95 respirators are worn by both the
patient and the healthcare personnel to protect from the transfer of microorganisms, body fluids,
- 7 -
and particulate material. An N95 respirator has headbands and is designed to achieve a very close
facial fit and very efficient filtration of airborne particles. If properly fitted, the filtration
capabilities of N95 respirators far exceed those of face masks and surgical masks. A picture of an
N95 respirator is attached below:
20. A NIOSH-approved N95 respirator is an N95 respirator approved by
NIOSH that meets the filtration efficiency level per 42 CFR 84.170. The NIOSH stamp of
approval is sought after by manufacturers and healthcare personnel alike as an indication of
safety, reliability, and performance.
21. NIOSH-approved respirators are required to have certain markings,
including the business name of the approval holder/manufacturer; NIOSH in block letters or the
NIOSH logo; the NIOSH testing and certification approval number, e.g., TC-84A-XXXX;
NIOSH filter series and filter efficiency level, e.g., N95; and the approval holder’s respirator
model number or part number, represented by a series of numbers or alphanumeric markings, e.g.,
8577 or 8577A. Below is a picture published by the CDC of a generic facepiece respirator with
appropriate NIOSH markings:
- 8 -
22. The CDC does not recommend that the general public wear N95 respirators
to protect themselves from respiratory diseases, including COVID-19, as those are critical
supplies that must be reserved for health care workers and other medical first responders.
23. Due to the unprecedented demand for emergency medical services to treat
patients presenting with COVID-19 symptoms, hospitals and medical professionals have
experienced critical shortages of N95 respirators and other one-time usage face masks.
The Statutory Framework
24. The FDA is responsible for protecting the health of the American public by
ensuring, among other things, that medical devices are safe and effective for their intended uses
and bear labeling that contains true and accurate information. The FDA, among other things,
regulates the manufacture, labeling, and distribution of medical devices shipped or received in
- 9 -
interstate commerce and enforces the Food, Drug, and Cosmetic Act, 21 U.S.C. §§ 301 et seq.
(the “FDCA”), and other pertinent laws and regulations.
25. The FDCA prohibits, among other things, the introduction, delivery for
introduction, or causing the introduction or delivery for introduction into interstate commerce of a
misbranded device. 21 U.S.C. § 331(a).
26. Under the FDCA, a medical device is misbranded if, among other things,
the labeling on the device “is false or misleading in any particular.” 21 U.S.C. § 352(a)(1).
27. Under the FDCA, a “label” is defined as “a display of written, printed, or
graphic matter upon the immediate container of any article.” 21 U.S.C. § 321(k). “Labeling” is
defined as “all labels and other written, printed, or graphic matter upon any article or any of its
containers or wrappers, or accompanying such article.” 21 U.S.C. § 321(m).
28. Under the FDCA, “interstate commerce” is defined in part as “commerce
between any State or Territory and any place outside thereof.” 21 U.S.C. § 321(b).
29. Under the FDCA, a “device” is defined, in pertinent part, as “an
instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar
or related article, including any component, part, or accessory, which is . . . (2) intended for use in
the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of
disease, in man . . . , and which does not achieve its primary intended purposes through chemical
action within or on the body of man or other animals and which is not dependent upon being
metabolized for the achievement of its primary intended purposes.” 21 U.S.C. § 321(h).
30. Face masks, face shields, and respirators are “devices” subject to FDA
regulation when they are intended for a medical purpose, such as prevention of infectious disease
- 10 -
transmission (including uses related to COVID-19). Face masks, face shields, and respirators are
not devices when they are intended for a non-medical purpose, such as for use in construction.
31. Under the FDCA, every person who owns or operates any establishment
within any foreign country engaged in the manufacture of a device that is imported or offered for
import into the United States shall, upon first engaging in such activity, immediately submit a
registration to FDA that includes, among other things, the name and place of business of the
establishment, the name of the United States agent for the establishment, and the name of each
importer of such device in the United States that is known to the establishment. 21 U.S.C. §
360(i)(1)(ii).
Individual-3 Agreed to Purchase N95 Respirators in the PRC from Individual-1
32. In or around March 2020, Individual-1, the American broker living in the
PRC, began sourcing PPE, including N95 respirators and COVID-19 test kits, for Individual-3,
the individual living in Pennsylvania and the owner of Company-2, from sources in the PRC.
Individual-3 intended to import and sell the products procured by Individual-1 to his customers in
the United States, including medical providers and first responders.
33. Individual-1 and Individual-3 agreed that they would use Individual-2’s
company, Company-1, to wire money to sources in the PRC and to help import the products into
the United States. Prior to the pandemic, neither Individual-2, the Utah-based acupuncturist, nor
Company-1 had experience in the PPE business.
34. From on or about March 18, 2020 to on or about March 27, 2020,
Individual-3 wired approximately $3.07 million to Company-1 for the purpose of purchasing 1
million N95 respirators. Individual-1 caused Company-1 to enter into separate agreements with
- 11 -
two Chinese companies in the PRC, Company-3 and Company-4, to purchase a total of
approximately 800,000 NIOSH-certified N95 masks for Individual-3 in the United States.
Individual-1 Procured the Subject Respirators from KING YEAR
for Individual-3 in the United States
35. On or about March 28, 2020, via text message, Individual-1 reported to
Individual-3 that he had obtained approximately 10,000 masks for Individual-3 and expected to
obtain an additional 20,000 to 50,000 masks the following day, which he intended to start
shipping to Individual-3 in the United States. Individual-1 sent Individual-3 pictures of the
packaging for each box of masks which listed the manufacturer as the defendant KING YEAR
PRINTING AND PACKAGING CO., LTD.
36. Individual-1 provided Individual-3 with a certificate of registration
document from East Notice Certification (“ENC”) service, dated March 20, 2020, and signed by a
purported Chief Engineer at ENC. Even though ENC was purportedly a Chinese inspection
company, the document had an FDA logo, an American eagle, and purported to state that the
defendant KING YEAR PRINTING AND PACKAGING CO., LTD. was “registered with the
U.S. Food and Drug Administration pursuant to voluntary cosmetic registration program.” A
picture of the certification document prepared for KING YEAR is attached below:
- 12 -
37. In fact, according to FDA records, the defendant KING YEAR PRINTING
AND PACKAGING CO., LTD. did not attempt to register with the FDA as a manufacturer of
N95 respirators until on or about April 10, 2020, not the March 20, 2020 date this certificate
bears. Therefore, this certificate prepared for KING YEAR by ENC and disseminated to
Individual-1 and others as a marketing tool for U.S. buyers, such as Individual-3, provided a false
impression that KING YEAR had registered with the FDA as a foreign manufacturer of N95
masks, when it had not.
38. Individual-1 also sent Individual-3, by text message, a test report prepared
for the defendant KING YEAR PRINTING AND PACKAGING CO., LTD. by ENC that
purportedly certified that the masks manufactured by KING YEAR met the NIOSH standard for
particulate filtration efficiency. The test report was from a non-accredited laboratory and appears
to have been false. The report did not include a model number or brand name for the masks
tested, rendering it impossible to verify whether the masks allegedly tested were the Subject
- 13 -
Respirators sold to Individual-3. Additionally, the pictures of the masks depicted in the report
were generic and appeared to be different from the Subject Respirators later imported by
Individual-3 into the United States. For example, unlike the Subject Respirators, the masks
depicted in the ENC report were not stamped with any marks or logos, were not embroidered with
“N95,” and had ear loops that visibly appeared to be made of different material.
KING YEAR Put False and Misleading Claims on the Labeling of the Subject Respirators
39. On or about April 6, 2020, the U.S. Customs and Border Protection
(“CBP”) intercepted and detained a shipment of approximately 95,200 Subject Respirators at the
John F. Kennedy Airport (“JFK”) that were inbound from the PRC and destined for Individual3’s warehouse in Newark, Delaware (“Shipment 1”). Shipment 1 consisted of approximately
4,760 identical boxes of masks, with each box containing approximately twenty masks.
40. Law enforcement reviewed pictures of the Subject Respirators and the
labeling on the packaging for the Subject Respirators. The labeling on one face of each box
stated “N95,” contained the logos of FDA and NIOSH, and claimed to meet the NIOSH filtration
standard. Another face of the box contained a warning label, listed the manufacturer as the
defendant KING YEAR PRINTING AND PACKAGING CO., LTD., and again prominently
displayed the NIOSH and FDA logos. The packaging also included the logos suggesting it
complied with European Union health laws and regulations (CE) and Chinese laws and
regulations (GB).
41. Each respirator had ear loops. One side of each respirator was embroidered
with “N95.” The other side was embroidered with “CE FFP2, EN149:2001+A1:2009,” which
refers to European Union filtration standards for respirators. Below are pictures of the labeling
on the Subject Respirators:
- 14 -
- 15 -
42. The labeling on the Subject Respirators manufactured by KING YEAR
was false and misleading because, among other things: (i) the packaging for the Subject
Respirators displayed the NIOSH logo prominently in two places, falsely implying the Subject
- 16 -
Respirators were NIOSH-approved (they were not); (ii) the labeling on the Subject Respirators
included the FDA logo, implying the Subject Respirators were FDA-approved, cleared, or
authorized (they were not); and (iii) the Subject Mask were embroidered with “N95” in their
fabric (they did not meet the N95 standard).
43. In addition, certifications prepared for the defendant KING YEAR
PRINTING AND PACKAGING CO., LTD. and disseminated to brokers, such as Individual-1,
falsely implied that KING YEAR was registered with the FDA as a manufacturer of N95 masks
(it was not registered until April 10, 2020, and the registration documents filed were misleading),
and that the Subject Respirators met the NIOSH filtration standard for N95s pursuant to a
purported lab test report prepared by ENC (ENC is not an accredited laboratory, and the CDC
later concluded that a sample of the Subject Respirators failed to meet the N95 filtration
standard).
Individual-3 Sold the Subject Respirators to First Responders and Medical Providers
44. Individual-3 marketed, priced, and sold the Subject Respirators as FDAapproved, NIOSH-approved, N95 respirators. In marketing materials sent to potential customers,
including healthcare providers, Individual-3 appended pictures of the false and misleading
packaging for the Subject Respirators containing the NIOSH and FDA logos, the false and
misleading ENC certification, and the false and misleading ENC test report.
45. Individual-3 then attempted to sell the Subject Respirators at prices
ranging from $4.85 to $5.85 per mask, to various entities, including, hospitals, pilots associations,
medical centers, and governments.
46. On or about April 6, 2020, a customs broker hired by Individual-3 to clear
Shipment 1 through U.S. customs raised questions concerning the intended use of the Subject
- 17 -
Respirators and whether they should include FDA and CBP authorization codes for importation.
Individual-3 relayed these questions over text message to Individual-1 in the PRC.
47. On or about April 8, 2020, Individual-1 advised Individual-3 in a text
message to disclaim the Subject Respirators as being for medical use because the masks were
“civilian use N95 masks.” Individual 3 then instructed the customs broker to disclaim the Subject
Respirators as intended for a medical use, notwithstanding the fact that Individual-3 intended to
sell the Subject Respirators to first responders and medical providers. As a result of this false
information, FDA did not perform an initial inspection of the Subject Respirators upon arrival at
JFK.
KING YEAR Filed False Registration Documents with the FDA
48. On or about April 10, 2020, the defendant KING YEAR PRINTING AND
PACKAGING CO., LTD. filed documents with the FDA to register as a foreign exporter and
manufacturer of “[f]ace Masks, KN95, N95, N95 respirator[s] with antimicrobial/antiviral
agent[s].” This was the first time KING YEAR filed a registration document with the FDA
concerning the Subject Respirators, notwithstanding that KING YEAR manufactured and offered
the Subject Masks for import into the United States as early as in or around March 2020.
49. The defendant KING YEAR PRINTING AND PACKAGING CO., LTD.
coded its products in the registration documents as Class II medical devices and “single use,
disposable, NIOSH-approved N95 respirator[s].” KING YEAR’s registration submission further
designated its respirators as “intended for use by the general public in public health medical
emergencies to cover the nose and mouth of the wearer to help reduce wearer exposure to
pathogenic biological particulates and has an added antimicrobial and/or antiviral agent which
kills specified pathogens under specified contact conditions.” This was false. First, KING YEAR
- 18 -
did not obtain NIOSH approval for the Subject Respirators. Instead, KING YEAR obtained a test
report from ENC, a non-accredited Chinese laboratory, falsely claiming that the Subject
Respirators satisfied the N95 standard. Second, the Subject Respirators were not intended to be
used by the general public. KING YEAR knew or consciously avoided knowing that any
respirators sold in the United States were going to be used first and foremost by healthcare
personnel on the frontlines of the COVID-19 health emergency given the widely reported
shortages of respirators in the national stockpile and in the U.S. market generally, and the CDC’s
guidance that all respirators should be reserved for healthcare personnel. By embroidering “N95”
on each individual mask and falsely marking the packages with the NIOSH logo on the
packaging, KING YEAR was actively causing the respirators to be most marketable to healthcare
personnel in the United States.
50. In the false registration documents filed by the defendant KING YEAR
PRINTING AND PACKAGING CO., LTD. with the FDA, it nominated CCTC Service, Inc.
(“CCTC”) as its U.S. agent, and listed an address for CCTC in Wilmington, Delaware, a phone
number, and an email address.
51. The responsibilities of the United States agent, include: assisting the FDA
in communications with the foreign establishment; responding to questions concerning the
foreign establishment’s devices that are imported or offered for import into the United States; and
assisting the FDA in scheduling inspections of the foreign establishment. 21 CFR 807.40(b)(2).
If the FDA is unable to contact the foreign establishment directly or expeditiously, the FDA may
provide information or documents to the U.S. agent, and such an action shall be considered to be
equivalent to providing the same information or documents to the foreign establishment. Id. In
addition, the United States agent cannot be a mailbox, answering machine or service, or any other
- 19 -
place where an individual acting as the foreign establishment’s agent is not physically present. 21
CFR 807.3.
52. The investigation revealed that the address listed by the defendant KING
YEAR PRINTING AND PACKAGING CO., LTD. for CCTC in its certification documents was
a personal residence with no affiliation to CCTC. Law enforcement interviewed the occupant of
the residence, who stated that he was a clinical psychologist who has rented the premises for three
years. The occupant had never heard of CCTC or KING YEAR. Law enforcement subsequently
interviewed the owners of the residence, both of whom stated that they had not heard of CCTC or
KING YEAR, and had never registered any company with the FDA.
53. On or about May 28, 2020, law enforcement attempted to reach out
multiple times to the phone number listed by the defendant KING YEAR PRINTING AND
PACKAGING CO., LTD. in its registration documents. Each time, law enforcement received a
message stating that the number was temporarily unavailable. Subsequent attempts to reach
anyone at that number were unsuccessful. CCTC has not responded to emails sent by law
enforcement to the email address listed for the company by KING YEAR on its registration
documents. Searches of open source records and law enforcement databases revealed no active
corporation by the name of CCTC in Delaware or elsewhere. There is, therefore, probable cause
to believe CCTC is a fictitious corporation.
54. In my training and experience, foreign manufacturers of counterfeit goods
use fictitious corporations as U.S. agents to circumvent regulatory oversight of their products.
The Subject Respirators Did Not Meet the Minimum N95 Standard
in Tests Performed by the CDC
55. On or about April 17, 2020, FDA employees reviewed photographs of the
first shipment of the Subject Respirators and found that they appeared to be misbranded.
- 20 -
56. On or about April 18, 2020, CBP intercepted a second shipment of the
Subject Respirators at JFK intended for delivery to Individual-3’s warehouse in Newark,
Delaware. This shipment consisted of 300,000 Subject Respirators (“Shipment 2”).
57. On or about April 22, 2020, the FDA performed an initial inspection of the
Subject Respirators at JFK from Shipments 1 and 2 and confirmed that they were misbranded.
On that same day, the FDA sent a sample of 10 masks from each shipment to the CDC for testing.
58. On or about April 22, 2020, a third shipment of the Subject Respirators
arrived at JFK inbound from the PRC and was intercepted by CBP (“Shipment 3”). Shipment 3
was also destined for Individual-3’s warehouse in Newark, Delaware. Law enforcement
confirmed that the packaging for the masks in Shipment 3 appeared to be identical to the Subject
Respirators in Shipments 1 and 2.
59. On or about April 24, 2020, the CDC published the results of the tests
performed on the Subject Respirators in Shipments 1 and 2. The CDC found that all of the tested
samples measured a filter efficiency of less than the minimum 95 percent required for an N95
respirator. The filter efficiency for the masks ranged from 83.4% to 91.12% for the first sample
and 82.7% to 90.03% for the second sample. With the exception of two masks, all of the masks
tested with a filter efficiency rate of below 90%. The CDC found that despite the packaging
having the NIOSH logo and claiming that the product met the NIOSH certification standard, the
Subject Respirators were not NIOSH-approved and did not meet the NIOSH certification
standard.
60. In addition, the CDC report noted fit difficulties with the ear loop design of
the Subject Respirators, stating that “[c]urrently, there are no NIOSH-approved products with ear
loops” and that “limited assessment of ear loop designs, indicate difficulty achieving a proper fit.”
- 21 -
The fit of a respirator is critical because it is only when an N95 respirator is properly fitted that its
filtration capabilities exceed those of face masks and surgical masks, and thereby, provide greater
protection from airborne particles and respiratory droplets containing the virus.
61. The FDA Center for Devices and Radiological Health (“CDRH”)
Inspections and Audits team also reviewed the Subject Respirators from Shipments 1 and 2. In
addition to CDC’s findings, CDRH concluded that the ENC test report prepared for KING YEAR
PRINTING AND PACKAGING CO., LTD. was not from an accredited laboratory.
62. WHEREFORE, your affiant respectfully requests that a summons be issued
for the defendant KING YEAR PRINTING AND PACKAGING CO., LTD. so that it may be
dealt with according to law.
Dated: Brooklyn, New York
June 5, 2020
Donald Pearlman
Special Agent
FDA Office of Criminal Investigations
Sworn to before me by telephone this
5th day of June, 2020
____________________________________
THE HONORABLE STEVEN M. GOLD
UNITED STATES MAGISTRATE JUDGE
EASTERN DISTRICT OF NEW YORK

文章来源于海运电商在线,仅供参考,如有侵权,请联系,内容属作者个人观点。不代表官方立场。

 巨东物流系统
巨东物流系统